April 10, 2017
Early in her career, Elodie Sollier-Christen, Ph.D. was trying to decide whether to study medicine or engineering when she learned about lab-on-a-chip concepts and recalls, “I found these concepts really exciting and I thought they would be the future of medicine.” Fast forward to 2017. The now chief scientific officer for Vortex Biosciences earned the 2017 SLAS Innovation Award winner for her presentation, “Classification of Large Circulating Tumor Cells Isolated with Ultra-High-Throughput Microfluidic Vortex Technology,” built from those early interests.
Sollier-Christen pursued her interest in lab-on-chip technologies by beginning an engineering program in biomedical engineering at Grenoble INP at Grenoble, France. “The program was recently developed and set up to use engineering sciences and microengineering for biological applications.”
Then, her Ph.D. work at CEA LETI Minatec focused on a miniaturized system for blood sample preparation, to separate the various components of the blood for different types of downstream assays. She had noticed vortices forming in a microfluidic channel and was using this phenomenon to extract plasma from whole blood. At the same time the impact of microvortices on cell separation was also being studied by Claire Soojung Hur, Ph.D., in the lab of Dino Di Carlo, Ph.D., at the University of California at Los Angeles (UCLA).
It was this shared interest in this unique phenomenon that brought Dr Sollier-Christen to join the Di Carlo lab.
Sollier-Christen explains that CTCs are of interest to researchers and oncologists because they may prove to be a surrogate for tissue biopsies and may be present in the blood prior to detection of the primary tumor. A liquid biopsy (from a blood draw) is cheaper, less invasive and less painful than obtaining a solid tissue biopsy. She adds that using the liquid biopsy may also help oncologists to monitor patients more frequently and make therapeutic adjustments based on the patient’s response.
As stated in a recent publication, “Circulating tumor cells (CTCs) are cancer cells that have been shed from a tumor into the bloodstream and play a major role in metastasis. The relative number of CTCs is predictive of patient prognosis and treatment efficacy.”
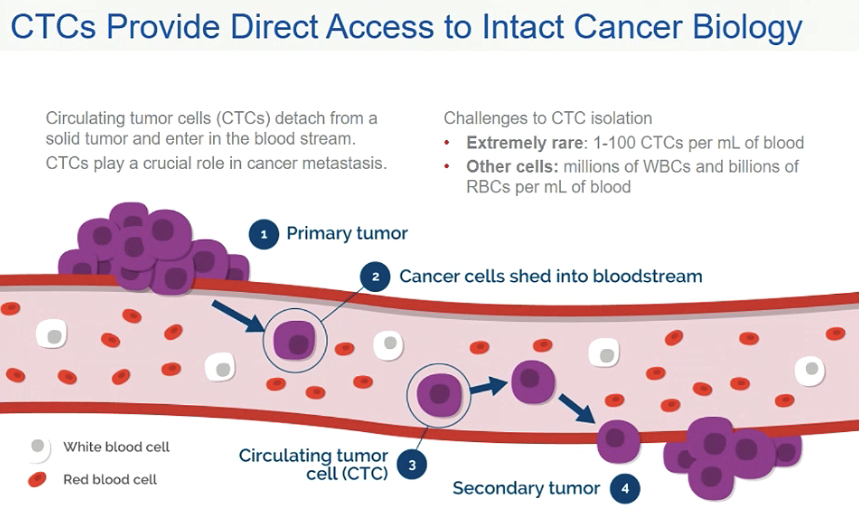
This article outlines the three key areas in which the isolation of CTCs from other blood elements can have a positive impact:
First, the number of CTCs has been shown to be predicitive of cancer prognosis and may also assist with characterizing treatment efficacy.
Second, CTCs can also be used in cell and molecular analysis, especially for personalized cancer therapies
Third, CTC isolation and analysis is crucial for cancer research: towards understanding mechanism in which secondary tumor sites form.
Sollier-Christen explains that “the way we see and approach cancer is changing. Clinicians currently rely on conventional tissue biopsies to diagnose the cancer, to assess the state of the disease and to decide what treatments will be given to the patient. However, tissue [needle] biopsies are painful, expensive, have a risk of complication and not all tumors are accessible. Additionally, we now know that cancer is very heterogeneous and the small biopsy may not be representative, giving the clinician incomplete information. Also, when the tumor is surgically removed, it is no longer available. The patient still has cancer, but you cannot check how the cancer is changing over time. A liquid biopsy, in contrast, involves a simple blood draw that is less painful, cheaper and can be repeated to monitor the patient for disease status. But to convince researchers, clinicians and patients of the usefulness of the CTCs, we need a technology that must be low cost, simple to use, reproducible and fast.”
Sollier-Christen is excited that the technology, while very sophisticated, has an apparent simplicity that is easy for people to understand. She says, “when you view the video on our website, it really shows what is happening in the chip in real time.” She thinks that “anyone who has walked by a river, and noticed places where there are whirlpools with leaves and branches stuck in the swirling water can see how fluids can accumulate objects in regions of vorticity. People get it very quickly; they can see objectively that it is very simple overall.”
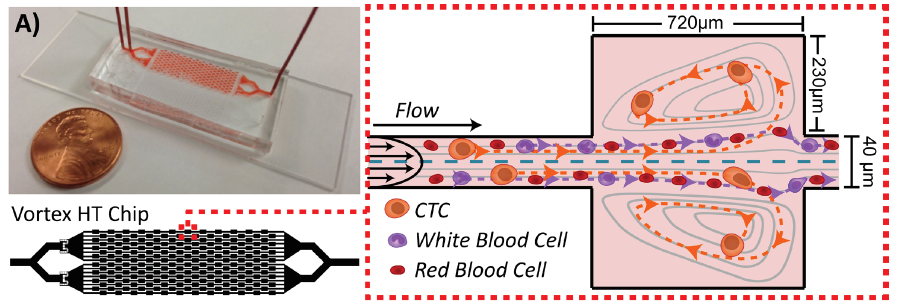
The early chip, tested on cancer patient blood in Di Carlo’s lab and described in this 2014 publication provided proof of concept and was able to achieve high purity but had a low capture efficiency of approximately 20 percent. Improvements would be needed for commercialization. This 2016 report describes the iterative improvements made to create a new high-throughput chip (Vortex HT) with its markedly increased processing speed and capture efficiency achieved by optimizing microchannel dimensions, improving flow control, and introducing serial re-processing. The schematic below depicts the Vortex HT with 2x more parallel channels and 1.5x more serial reservoirs. The HT chip used in the VTX-1 instrument retains high purity (< 100 WBCs/mL) while also increasing capture efficiency (>70 percent).
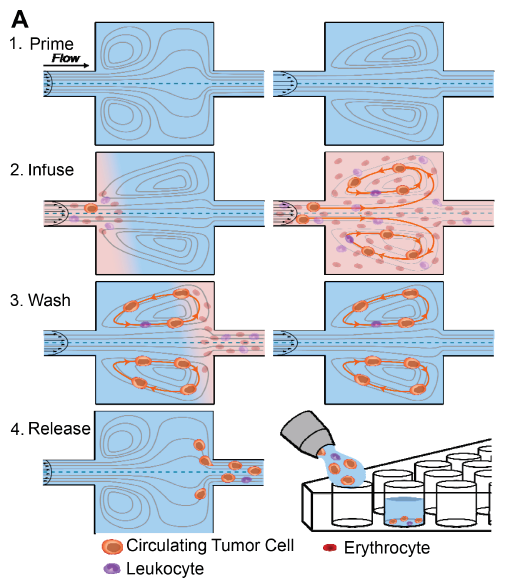
By using a sequence of steps described in this paper and visually represented below, the CTCs are first isolated from the other blood elements at a high flow rate. The CTCs become trapped in the cavities as smaller elements (red and white blood cells) continue to flow downstream. The CTCs are then washed while remaining trapped in a buffer solution and finally, they are released by lowering the flow rate. The CTCs can be collected into a well-strip or other containers for downstream analysis.
The core of the liquid biopsy system is the Vortex chip that isolates CTCs based on the cell’s physical characteristics of size and deformability rather than labeling the cells with a marker molecule. Sollier-Christen explains this is important because not all cancer cells express the expected surface molecules needed for binding to an antibody or other labeling molecule.
Sollier et al. also report that other alternatives for isolating CTCs relying on cell properties rather than membrane protein expression are being developed. The authors describe the systematic testing of the fluid physics, optimization of the Vortex HT chip design and validation of the new system with cancer patient blood. The methodology is gentle enough on the cells that viable CTCs from patient blood are obtained and can be used for research inquiring about the cancer cell’s biology.
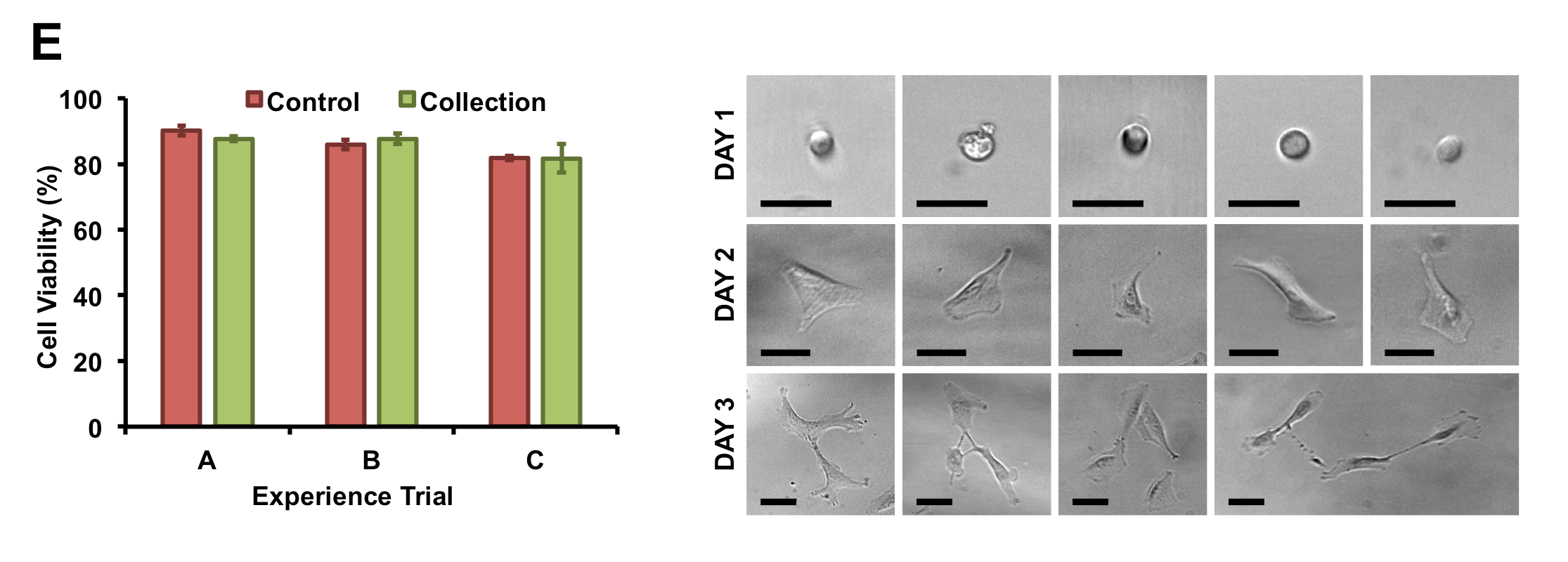
Image used with permission from Lab on a Chip 2014.
The fast, easy, cheap and reproducible criteria have driven the design of the recently released Vortex VTX-1 Liquid Biopsy System using a newly designed chip, automated fluid handling and optimized workflows. Explained in her SLAS2017 presentation, the instrument user can directly connect a blood collection tube to a plastic cartridge which is loaded into the VTX-1 and in one hour, isolated CTCs are collected into commonly used containers for downstream analysis.
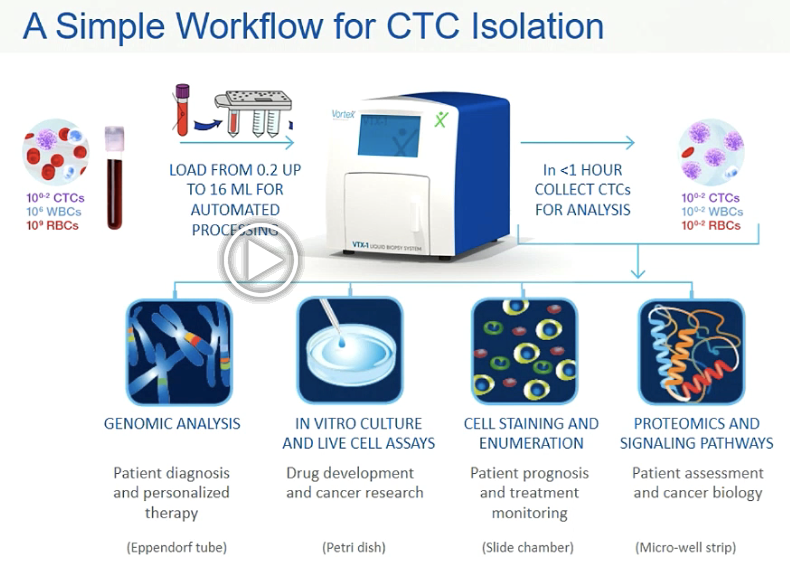
The VTX-1 instrument was launched shortly after SLAS2017 and will be initially marketed to researchers. Sollier-Christen explains that the Vortex Biosciences team has plans in two main areas for the next evolution of the technology.
The first is the development of clinical assays. The team is collaborating with researchers to explore and create validated assays that use the isolated CTCs as a next step in patient care. Current projects are looking at EGFR mutations in lung cancer and also at in vitro expression of markers important for immunotherapy. She explains that part of this work will be to demonstrate that performing these clinically relevant tests on CTCs is equivalent to using conventional tissue biopsy specimens. “If these clinical studies achieve the results we think they will, it opens the door for how CTCs can be used in the clinic,” says Sollier-Christen.
The second is to develop the instrument further, adding functions to the basic platform through research collaborations. Sollier-Christen mentions that Hur is exploring “vortex electroporation” which will create pores in captured cells to allow the introduction of drugs, DNA or proteins. In Di Carlo’s lab, they are trying to count the CTCs while they are being released to have a real-time estimate of the extent of the disease and to inform clinicians as they plan either additional diagnostics or changes in therapeutic protocols.
Sollier-Christen mentions that there are a number of challenges for this technology in both research and clinical settings and feels that one big question to be explored is the use of CTCs for earlier cancer stages (pre-metastasis). There are very few studies using CTCs to detect cancer before a primary mass is detected, and there is a need for clinical studies in this area.
Very early in the development of the Vortex technology, strong partnerships were developed with oncology clinicians, Dr. Jonathan Wade Goldman from UCLA and Dr. Stefanie S. Jeffrey from Stanford University. Because of these collaborations, the Vortex team has been able to work with samples from patients with colorectal, lung and breast cancer. Sollier-Christen feels strongly that access to blood from cancer patients and age-matched controls are great assets in testing and validating Vortex’s technology.
Sollier-Christen has a personal interest in the diagnosis and treatment of cancer and finds this passion very common among the people in her company and others working in the oncology realm. “I know Vortex Biosciences would not be where we are today without supportive people from the beginning,” she says. “Of course, you need a strong technology, a good market, and the technology needs to answer a crucial need. But you must have a strong team.”
Early investors enabled Sollier-Christen and Di Carlo to bring on people with the skills and experience needed to make a successful commercial product. “The success and rapid development of this technology is due to having a very focused and excellent team working together, Sollier-Christen says. Gene Walther, former President of Chiron Blood Testing and Novartis Diagnostics, joined Vortex Biosciences in January 2016. Steve Crouse, coming from Life Technologies and Biorad, joined to head the commercial organization, while Bob Englert, with extensive experience at Abbott Laboratories and medical device start ups, joined to lead the product development and manufacturing.
Sollier-Christen sees SLAS Technology Reviews Editor Di Carlo as a perfect example of a brilliant scientist who is really interested in seeing his research applied to the benefit of end users. In 2012, Di Carlo and Sollier-Christen founded Vortex Biosciences to commercialize the technology. As chief scientific officer of Vortex, Sollier-Christen is thrilled about the SLAS Innovation Award as recognition of the team’s efforts. “This is further validation of the breakthrough technology the Vortex VTX-1 Liquid Biopsy System is and the impact this will have on cancer patients,” she says.
SLAS2017 was the first society conference that Sollier-Christen has attended and she found it unlike any other she has experienced. She is used to conferences focused on very early stage research but the SLAS meeting is more about end results and real solutions. She enjoyed being able to see how microengineering technologies were being transferred from the bench to real commercial products.” It’s great because it shows a different aspect of research work,” she says. “I think more Ph.D. students should go to this type of conference to see the reason why they do the research.”
Sollier-Christen’s SLAS2017 presentation, “Vortex Biosciences Technology for Fast and Label-Free Isolation of Circulating Tumor Cells from Blood Samples,” provides a detailed explanation of the technology and its applications. It is available as an on-demand webinar (synched slides and audio) free-of-charge to SLAS members always and to non-members through the end of April 2017.
Size-Selective Collection of Circulating Tumor Cells using Vortex Technology
Non-invasive Trapping and Imaging of Circulating Tumor Cells