
March 5, 2018
“We are pleased to recognize the innovators behind the 2018 SLAS Technology Ten,” says Edward Kai-Hua Chow, SLAS Technology editor-in-chief (National University of Singapore). “These hard-working individuals from Australia, China, Poland, Singapore, Spain, Switzerland, Taiwan and the United States had breakthroughs in micro-, nano- and digital technologies to improve drug delivery and therapy against a wide range of diseases, from wound healing to cancer. They report great advances in microfluidics, diagnostics, microtechnology, life sciences and biomedical assays and other technologies that are changing the way drugs are developed and evaluated to improve both efficacy and safety.”
A Wound-Healing Assay Based on Ultraviolet Light Ablation
By Shang-Ying Wu, Yung-Shin Sun, Kuan-Chen Cheng, Kai-Yin Lo

“Wound healing assays are an important technique for drug screening and to study cell migration behavior,” says Kai-Yin Lo, Ph.D., associate professor in the Department of Agricultural Chemistry at National Taiwan University (Taipei, Taiwan). “Development of a high-throughput, cheap and quick method is required. In this paper, we report using UV light with masks to create wounds.” The work continues as the lab also is working with sticker assays – cutting with a laser scriber to create wounds of different shapes and geometries.
Lo graduated from the University of Texas at Austin, with a Ph.D. in molecular genetics and microbiology. Her postdoctoral training was at the University of California, Davis. Her National Taiwan University lab now focuses on ribosome synthesis pathway and cell migration assays.
Engineering A11 Minibody-Conjugated, Polypeptide-Based Gold Nanoshells for Prostate Stem Cell Antigen (PSCA)–Targeted Photothermal Therapy
By Kristine M. Mayle, Kathryn R. Dern, Vincent K. Wong, Kevin Y. Chen, Shijun Sung, Ke Ding, April R. Rodriguez, Scott Knowles, Zachary Taylor, Z. Hong Zhou, Warren S. Grundfest, Anna M. Wu, Timothy J. Deming, Daniel T. Kamei

Daniel T. Kamei, Ph.D., is a professor in the Department of Bioengineering at the University of California, Los Angeles (Los Angeles, CA), and his molecular and cellular engineering research focuses on paper-based diagnostics and drug delivery. “We performed this study because we wanted to contribute to the fight against cancer,” he says of his SLAS Technology paper. “To do so, we combined a novel targeting ligand with a novel drug delivery material. This ligand had been used for positron emission tomography imaging but never for drug delivery, while the material had been used to deliver chemotherapeutics. The merging of these two technologies, combined with the added feature of coating the material with gold and using near-infrared light for excitation, led to the development of our nanoparticle system that targeted and killed prostate cancer cells.”
Kamei hopes readers learn from his team’s paper that mathematical modeling can play a significant role in engineering new therapies. “This is especially important when many processes are occurring simultaneously and are integrated in such a way that they all influence each other,” he adds. “Under these circumstances, intuition is not enough to determine how to design a technology, and a quantitative description becomes necessary. This is even more true when quantitative outputs, like a target temperature, are of interest, rather than just a qualitative on/off or increase/decrease output.”
A Smartphone-Based Genotyping Method for Hepatitis B Virus at Point-of-Care Settings
By Huiqin Jiang, Di Wu, Liuwei Song, Quan Yuan, Shengxiang Ge, Xiaoping Min, Ningshao Xia, Shizhi Qian, Xianbo Qiu
Personal interest in biomedical engineering and engaging academic training led author Xianbo Qiu, Ph.D., to his current position as professor at the Department of Automation, School of Information Science and Technology at Beijing University of Chemical Technology (BUCT), and head of the BUCT Microfluidic Biomedical Research Center (Beijing, China).
“Traditional HBV genotyping methods based on nucleic acid analysis are normally time-consuming and high cost due to the complicated procedures including sample processing, nucleic acid amplification, labeling, detection and analysis,” he explains. “Simplified methods are highly desired to extend the application of HBV genotyping especially at resource-poor settings. Our collaborative team from Xiamen University, Old Dominion University and Beijing University of Chemical Technology worked together to complete this multidisciplinary research project to provide a simple, easy-to-operate and low-cost HBV genotyping method. Our final goal is to put the developed method into real use in the future to benefit people, especially with limited medial resources.”
Optimizing Combination Therapy for Acute Lymphoblastic Leukemia Using a Phenotypic Personalized Medicine Digital Health Platform: Retrospective Optimization Individualizes Patient Regimens to Maximize Efficacy and Safety
By Dong-Keun Lee, Vivian Y. Chang, Theodore Kee, Chih-Ming Ho, Dean Ho

Dean Ho is a professor at the University of California, Los Angeles (UCLA) School of Dentistry and co-director of The Jane and Jerry Weintraub Center for Reconstructive Biotechnology (Los Angeles, CA). His recent work looks at ways where their novel artificial intelligence platform could actionably treat patients for a broad spectrum of diseases by both selecting the right therapies and modulating how these drugs are administered to continuously optimize patient care for the entire duration of treatment.
“After patients undergo treatment in oncology, their physiology changes constantly, and it is vital to continuously re-identify the optimal way to administer combination therapy,” Ho states. “Our technology platform successfully addresses this major challenge. We've helped patients respond more effectively to their treatment compared to conventional protocols. It is great to see patients resuming an active lifestyle.”
Ho notes the team has many clinical trials underway worldwide, and hopes readers appreciate that “the intersection of our augmented AI and patient care is expected to transform healthcare in the areas of technology, economics, policy/regulatory, etc.”
Layer-by-Layer 3D Constructs of Fibroblasts in Hydrogel for Examining Transdermal Penetration Capability of Nanoparticles
By Xiaochun Hou, Shiying Liu, Min Wang, Christian Wiraja, Wei Huang, Peggy Chan, Timothy Tan, Chenjie Xu

Chenjie Xu, Ph.D., is an assistant professor of bioengineering at Nanyang Technological University (Singapore). His research interest lies at the development of micro and nano-technologies for transdermal drug delivery. “The public has generously supported academic research in the past 50 years, and it is our duty to pay back to society,” he says. “In the biomedical field, one of the most favored drug administration methods is topical delivery, which is also the major way for cosmetic application. Due to its non-invasiveness and convenience, people like to choose products or drug formulations under this form.”
His work focuses on keloid, a type of abnormal scar. He chose this direction due its growing occurrence in Asia and Africa. “Due to the scarcity [of keloid] in Western countries, there has been less interest to develop technologies to target this problem. Based on my background, I have been building platform technologies for the diagnosis and treatment of keloid. This paper reports an effort in developing an in vitro model for screening formulations that can be used to deliver drugs. I want readers to know that 3D printing allows researchers to build platforms in labs for screening and testing the transdermal ability and biosafety of drug formulation.”
Microfluidic Tissue Mesodissection in Molecular Cancer Diagnostics
By Christine Surrette, David Shoudy, Alex Corwin, Wei Gao, Maria I. Zavodszky, Stanislav L. Karsten, Todd Miller, Michael J. Gerdes, Nichole Wood, John R. Nelson, Chris M. Puleo

“Our group at GE is driven to develop technologies that creatively disrupt workflows across the healthcare life sciences and diagnostics space,” says Christopher Puleo, Ph.D., (Niskayuna, NY). “We are always looking for ways in which technology can make these workflows simpler, more precise or more repeatable. In this paper, we discuss our simple microfluidic device that enables rapid mesodissection of tissue samples from formalin-fixed paraffin embedded (FFPE) slides in a way that is much more precise and automated compared to manual methods. The device only functions because of careful design of both the physical microfluidic component, and the custom fluid dissection reagent.
Puleo reports that during their work, they became impressed by a simple microscope dissection platform from a company called NeuroInDx. “At the time, the NeuroInDx systems worked for dissection of several tissues (including fresh frozen and live cells), but did not allow FFPE dissection,” he says. “We decided to test our FFPE dissection device on both an internal GE prototype and the flexible and uniquely-designed NeuroInDx platform. This technology’s impact to cancer research and diagnostics motivates us to put our time and talent behind this work. Our platform provides an affordable and fast workflow that provides clinical laboratories and researchers the ability to precisely isolate and extract a region of interest without being laborious.”
Heart-on-a-Chip: An Investigation of the Influence of Static and Perfusion Conditions on Cardiac (H9C2) Cell Proliferation, Morphology, and Alignment
By Anna Kobuszewska, Ewelina Tomecka, Kamil Zukowski, Elzbieta Jastrzebska, Michal Chudy, Artur Dybko, Philippe Renaud, Zbigniew Brzozka

Elzbieta Jastrzebska, Ph.D., is assistant professor at Warsaw University of Technology (Warsaw, Poland). She is committed to her research. “Heart disease is one of the main causes of death around the world,” she states. “Therefore, I would like to solve a small part of this problem and develop new tools and heart cell culture models which could improve the therapeutic methods for these types of ailments.
“In our SLAS Technology paper, we show that the external stimulation (continuous medium flow) enhanced both the growth and parallel orientation of cardiac (H9C2) cells. Additionally, we observe that these parameters depend on the geometry of the culture microchamber. Our results show that the proposed heart-on-a-chip can be utilized to understand processes in heart tissue in detail and to test newly developed compounds used for the treatment of cardiac diseases.”
Rapid Prototyping of a Cyclic Olefin Copolymer Microfluidic Device for Automated Oocyte Culturing
By Miguel Berenguel-Alonso, Maria Sabés-Alsina, Roser Morató, Oriol Ymbern, Laura Rodríguez-Vázquez, Oriol Talló-Parra, Julián Alonso-Chamarro, Mar Puyol, Manel López-Béjar
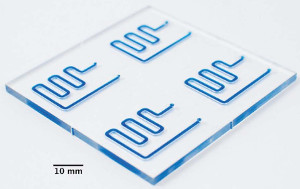
Analytical chemist Mar Puyol, Ph.D., is a lecturer at the Autonomous University of Barcelona from the Microfluidics and Integrated Analytical Microsystems for (Bio)Chemical Sensing Group, within the Sensors and Biosensors Group (Bellaterra, Spain). Her research interests include the study and characterization of new chromo(fluoro)ionophores and its integration as recognition elements in miniaturized optochemical sensors.
“This SLAS Technology paper shows an interesting fabrication approach based on the combination of two manufacturing methods, micromilling and hot-embossing, in order to allow the construction of different sized patterns required to retain the oocytes,” she explains. “Moreover, we want to highlight the great potential of the COC (cyclic olefin copolymer) for the development of microfluidic platforms for assisted reproductive techniques, including the possibility to integrate temperature control. This will avoid the use of other lab instrumentation such as incubators other controlling temperature systems.”
An Electrochemical Biosensor for Rapid Detection of Pediatric Bloodstream Infections
By Eranda M. K. Kurundu Hewage, Debbie Spear, Todd M. Umstead, Sanmei Hu, Ming Wang, Pak Kin Wong, Zissis C. Chroneos, E. Scott Halstead, Neal Thomas

“I grew up in Sri Lanka, a beautiful island in South Asia, where certain infectious diseases are relatively common,” says Eranda M.K. Kurundu Hewage B.VSc. (Hon.), M.S., research technologist II at the Pennsylvania State University College of Medicine (Hershey, PA). “As a child, I experienced how people suffer due to infectious diseases, and was amazed by how people in medical profession work together to save lives with limited resources. This inspired me to explore opportunities in which I can be involved in infectious diseases diagnosis and prevention as well as to search for new diagnostic methods for improved patient care.”
Hewage says the overall goal of the study in SLAS Technology was to evaluate the appropriateness of an electrochemical biosensor for detection of bloodstream infection (BSI) in pediatric blood. “In a previous proof of concept study, the efficiency of this method was confirmed with human blood,” he reports. “However, there was no data on comparison between adult and pediatric blood using this detection technology. It is crucial to acknowledge the possible variations between adult and pediatric blood matrices and probable differences of bacterial growth and to illuminate the effectiveness of the electrochemical biosensor for detection of bloodstream infections in pediatric blood. We were able to confirm that matrix effects of adult and pediatric blood toward growth of bacteria was very similar indicating that this technology is suitable for detection of BSI in pediatric blood.”
Adaptation of a Simple Microfluidic Platform for High-Dimensional Quantitative Morphological Analysis of Human Mesenchymal Stromal Cells on Polystyrene-Based Substrates
By Johnny Lam, Ross A. Marklein, Jose A. Jimenez-Torres, David J. Beebe, Steven R. Bauer, Kyung E. Sung
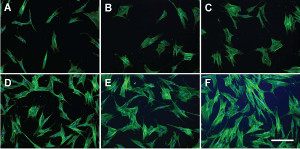
“Multipotent stromal cells (MSCs, often called mesenchymal stem cells) have garnered significant attention within the field of regenerative medicine because of their purported ability to differentiate down musculoskeletal lineages,” according to the paper’s abstract. “Given the inherent heterogeneity of MSC populations, recent studies have suggested that cell morphology may be indicative of MSC differentiation potential. Toward improving current methods and developing simple yet effective approaches for the morphological evaluation of MSCs, we combined passive pumping microfluidic technology with high-dimensional morphological characterization to produce robust tools for standardized high-throughput analysis.”
Corresponding author Kyung E. Sung, Ph.D., is a principal investigator, Center for Biologics Evaluation and Research, United States Food and Drug Administration (Silver Spring, MD). This work was carried out by his team in collaboration with David J. Beebe, Ph.D., in the Department of Biomedical Engineering at the University of Wisconsin, Madison.

“As editor-in-chief, I thank the 83 authors who contributed to the 2018 SLAS Technology Ten for their hard work, ingenuity and willingness to stretch the boundaries of what is possible in life sciences and biomedical technology,” Chow says. “I also thank the hundreds of other authors who also contributed great work to SLAS Technology in 2017 and the hundreds of manuscript reviewers who worked hard to maintain publication standards. The members of the SLAS Technology Editorial Board and I look forward to presenting more great work in the coming year.